LifeBridge Innovations began with the mission to save one life—and led to a breakthrough with the potential to impact millions.
Laurie M. Travers had battled Stage IV breast cancer through nine recurrences over 15 years. By late 2012, the cancer had spread aggressively to the pleura—the lining around her lung—causing severe fluid buildup that compressed her left lung and made it difficult to breathe. Every three weeks, she underwent thoracentesis to drain between 900 and 1,500 cc of fluid. Her cancer had become persistent and unresponsive to most treatments. Laurie and her family feared the end was near.
Click on image to view larger
During this time, Laurie’s husband, Peter, learned about a new FDA-approved therapy called Tumor Treating Fields, which had recently been approved for the treatment of glioblastoma. However, he quickly discovered that it wouldn’t be available to patients like Laurie for the foreseeable future. Determined to help his wife, Peter assembled a team of senior engineers to build a device themselves as a final act of hope. After hundreds of hours of intensive work, they successfully built a Tumor Treating Fields device that met the standards of the available research at the time. With Laurie’s treating oncologist providing close oversight, the TTF therapy was incorporated into her treatment protocol.
Amazingly... it worked.
Letter from Dr. Lee Zehngebot, M.D.
A firsthand clinical perspective on Laurie Travers’ response to TTF from her treating oncologist
“I am Dr. Lee Zehngebot, a medical oncologist. I cared for Laurie Travers from her initial breast cancer diagnosis through the final stages of her illness. Laurie was originally diagnosed with Stage II breast cancer in 1997. She had positive lymph nodes and was Her-2 Neu positive—unfortunately, this was prior to the availability of Herceptin. In 1998, she developed metastatic disease. Over the next 10+ years, she underwent multiple lines of treatment, entering and relapsing from remission several times. By late 2012, treatment options were running out. Laurie was accumulating 900–1500 cc of pleural fluid every three weeks, which had to be removed via thoracentesis. We attempted to reintroduce a previously successful chemotherapy. While scans showed improvement in the pleural disease, new asymptomatic tumors appeared on the top of her liver. Given these mixed results and rising tumor markers, I considered the chemotherapy a failure. It was at this point that I learned Peter Travers and his engineering team had developed their own Tumor Treating Fields (TTF) device and were applying it to Laurie’s left lung.
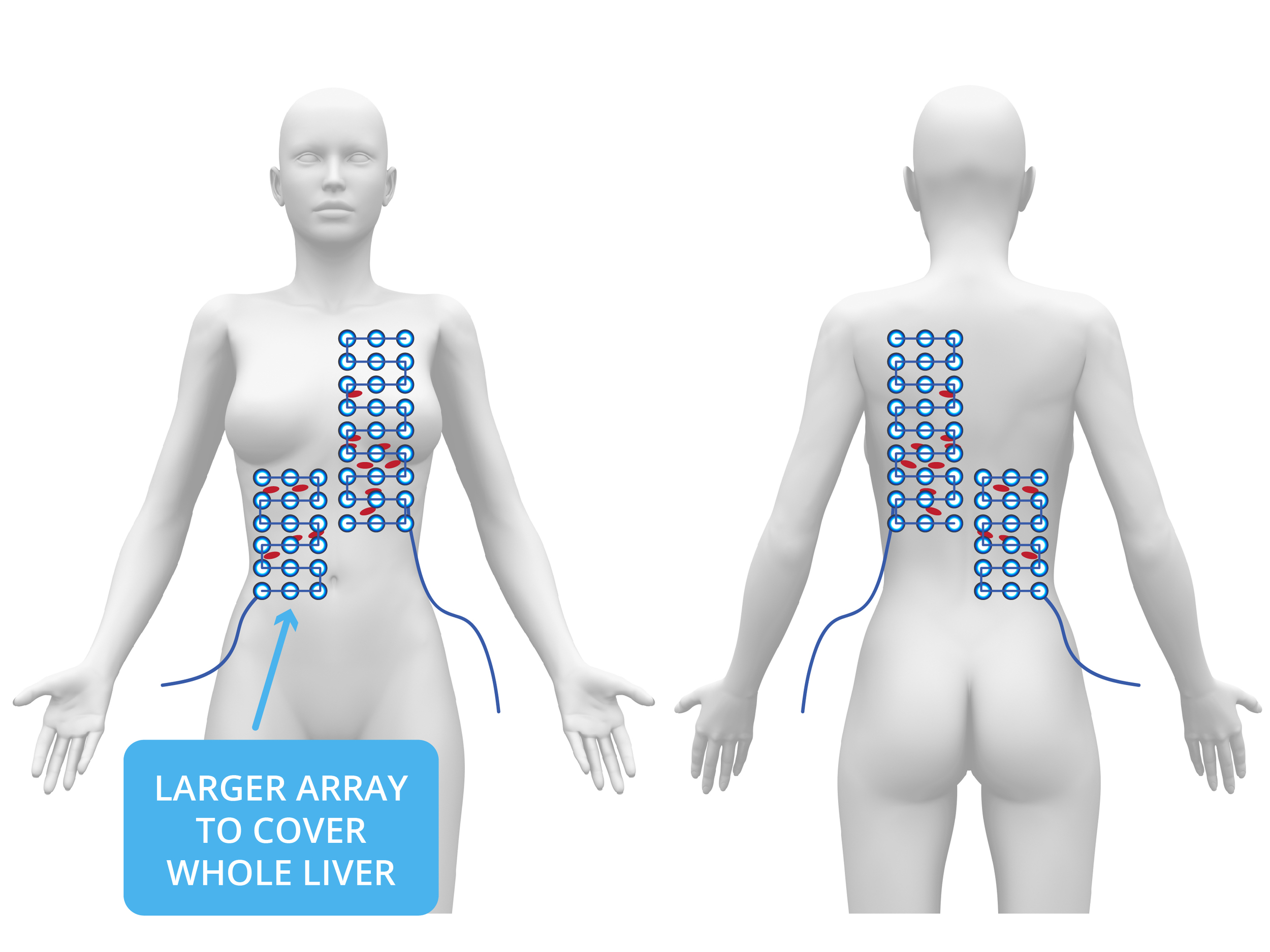
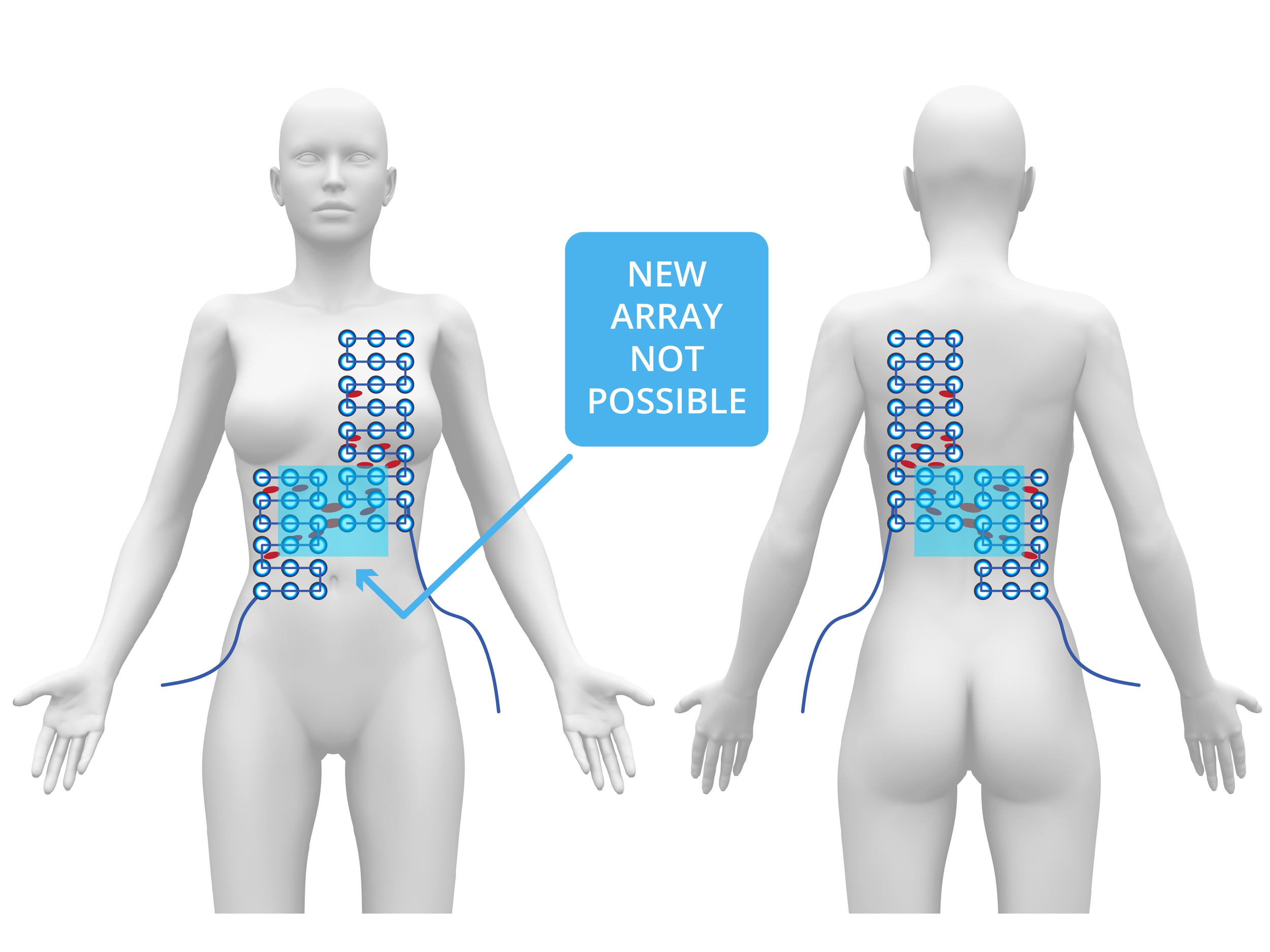
I resumed chemotherapy while Peter's team built a small TTF array to treat the liver. Subsequent scans revealed continued improvement in the pleura and now also in the top of the liver—but new tumors appeared on the underside of the liver. It became clear that remission was occurring only where TTF was being applied. We stopped all chemotherapy and focused solely on TTF therapy. I identified the liver treatment area, and Peter's team rapidly built a larger array to cover the full liver. TTF therapy was then used as monotherapy on the liver, while maintaining pleural coverage. Over the following months, the cancer in Laurie’s liver fully resolved, her tumor markers normalized, and she returned to work as a neonatal nurse—free from chemotherapy and supported only by maintenance TTF. In early 2016, tumor markers began rising again. New disease appeared in the peritoneal cavity. Unfortunately, the existing TTF device couldn’t adequately cover such diffuse areas.
I recommended continuing treatment to the liver, but the cancer progressed into the peritoneal cavity and deeper into both lungs. Peter later explained that their device couldn't be adapted to treat those deep lung regions due to heat issues from higher power requirements. With no effective options remaining, Laurie declined further treatment and passed away in mid-2016. I remain confident that the two-plus years of extended and relatively high-quality life Laurie experienced were directly attributable to TTF therapy.”
- Lee Zehngebot, M.D.
While first-generation Tumor Treating Fields could not save Laurie’s life, the therapy allowed her to gain precious time with her loved ones.
One year after the onset of severe cancer in the pleura., Laurie celebrated a personal milestone—her “Sweet 16”—marking 16 years of surviving cancer. Surrounded by loved ones, she gave thanks for the journey and the time she had. Laurie shared her reflections in a heartfelt note to her friends.
“Peter & I have spent the whole last week just in awe of God’s goodness & the love of friends. The joy of the celebration has lingered in our (heart emoji)’s. Considering how desperate life looked with each drain of my lung last year at this time - God’s miraculous turn-around is awesome. I feel so blessed to have been given more life to live -”
Laurie’s sacrifice exposed the limitations of first-generation TTF therapy, but showed the incredible potential of the treatment to battle diffuse metastatic disease… if only the treatment could reach every tumor.
Determined to honor Laurie’s legacy, the team at LifeBridge Innovations is advancing the next-generation of TTF therapy— Adaptive Tumor Treating Fields (ATTF). Designed from the ground up for late-stage cancer patients, our LB10000 device harnesses ATTF to treat metastatic disease in multiple areas of the body simultaneously with enhanced coverage and treatment dosage capabilities relative to legacy systems.